The Element Francium
[Click for Isotope Data]
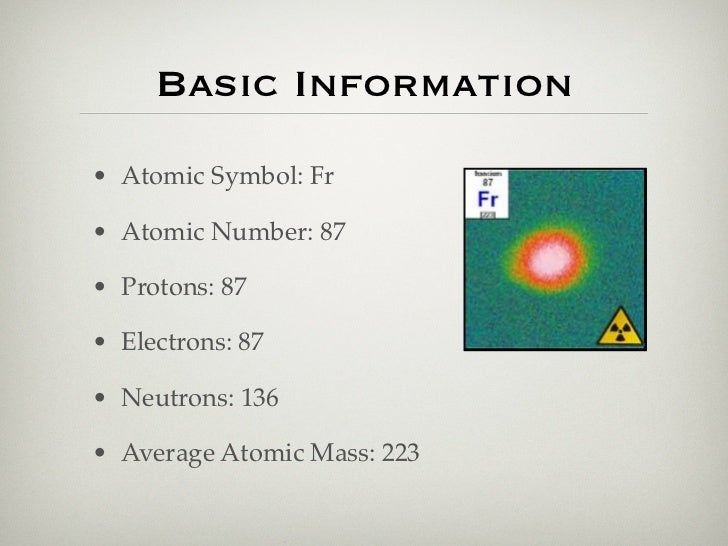
Atomic Number: 87
(The atomic number decreases by 2) If the radioelement emits Beta particles, the resulting elements must be placed one period to the right. (The atomic number increases by 1) The element that was two periods to the right of Francium is Actinium and the element on the left-hand side of Francium is Radon. Francium Page One. Overview of Francium; Francium's Name in Other Languages; Atomic Structure of Francium; Chemical Properties of Francium; Physical Properties of Francium; Regulatory / Health; Who/When/Where/How. Francium Page Two. Nuclides / Isotopes; Potential Parent Nuclides. Overview of Francium. Atomic Number: 87; Group: 1. French chemist Marguerite Perey discovered francium (1939) while studying actinium -227, which decays by negative beta decay (electron emission) to an isotope of thorium (thorium-227) and by alpha emission (about 1 percent) into an isotope of francium (francium-223) that was formerly called actinium K (AcK) and is a member of the actinium decay series. Francium can be produced by bombarding thorium with protons or by bombarding radium with neutrons. Francium's most stable isotope, francium-223, has a half-life of about 22 minutes. It decays into radium -223 through beta decay or into astatine -219 through alpha decay. CAS Registry: 7440-73-5 Francium atoms have 87 electrons and the shell structure is 2.8.18.32.18.8.1. The ground state electronic configuration of neutral francium is Rn. 7s1 and the term symbol of francium is 2S1/2.
Atomic Weight: 223
Melting Point: 300 K (27°C or 81°F)
Boiling Point: Unknown
Density: Unknown
Phase at Room Temperature: Solid

Element Classification: Metal
Period Number: 7
Group Number: 1
Group Name: Alkali Metal
Special Notes: Radioactive
What's in a name? Named for the country of France.
Say what? Francium is pronounced as FRAN-see-em.
History and Uses:
Formula For Francium

Francium was discovered by Marguerite Catherine Perey, a French chemist, in 1939 while analyzing actinium's decay sequence. Although considered a natural element, scientists estimate that there is no more than one ounce of francium in the earth's crust at one time. Since there is so little naturally occurring francium on earth, scientists must produce francium in order to study it. Francium can be produced by bombarding thorium with protons or by bombarding radium with neutrons.
Francium's most stable isotope, francium-223, has a half-life of about 22 minutes. It decays into radium-223 through beta decay or into astatine-219 through alpha decay.

/GettyImages-517345224-e456b6c739d64be89a519da75bb63e06.jpg)
Due to the small amounts produced and its short half-life, there are currently no uses for francium outside of basic scientific research.
Estimated Crustal Abundance: Not Applicable
Estimated Oceanic Abundance: Not Applicable
Number of Stable Isotopes: 0 (View all isotope data)
Ionization Energy: 3.9 eV
Oxidation States: +1
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 3d10 | |
4s2 4p6 4d10 4f14 | |
5s2 5p6 5d10 | |
6s2 6p6 | |
7s1 |
For questions about this page, please contact Steve Gagnon.
Francium Metal
|
View Nuclear Periodic Table | ||||||||||||||||||||||||||||||
History
Francium Atomic Number
(France) Discovered in 1939 by Mlle. Marguerite Perey of the Curie Institute, Paris. Francium, the heaviest known member of the alkali metals series, occurs as a result of an alpha disintegration of actinium. It can also be made by artificially bombarding thorium with protons. While it occurs naturally in uranium minerals, there is probably less than an ounce of francium at any time in the total crust of the earth. It has the highest equivalent weight of any element, and is the most unstable of the first 101 elements of the periodic system. Thirty-three isotopes of francium are recognized. The longest lived 223Fr (Ac, K), a daughter of 227Ac, has a half-life of 22 min. This is the only isotope of francium occurring in nature. Because all known isotopes of francium are highly unstable, knowledge of the chemical properties of this element comes from radiochemical techniques. No weighable quantity of the element has been prepared or isolated. The chemical properties of francium most resemble cesium.
