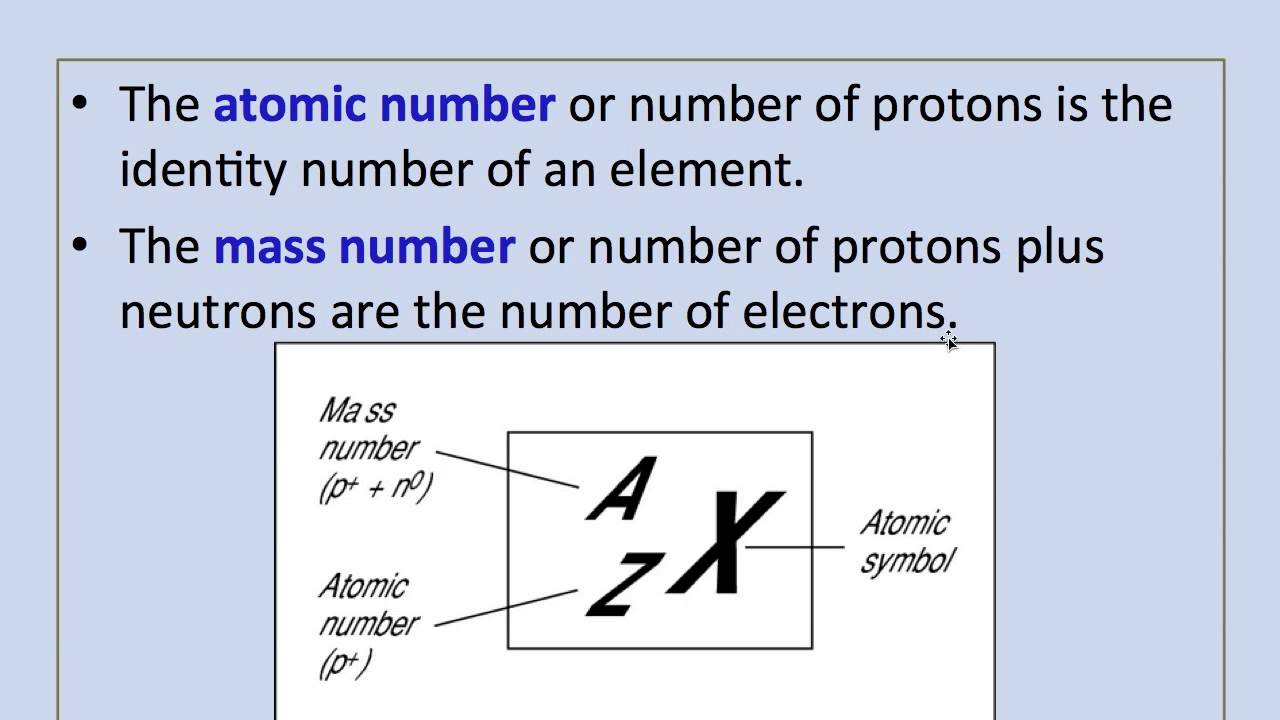
Atomic Number of Nickel is 28.
Nickel (atomic number 28) resembles iron (atomic number 26) in strength and toughness but is more like copper (atomic number 29) in resistance to oxidation and corrosion, a combination accounting for many of its applications. Nickel has high electrical and thermal conductivity.
Chemical symbol for Nickel is Ni. Number of protons in Nickel is 28. Atomic weight of Nickel is 58.6934 u or g/mol. Melting point of Nickel is 1453 °C and its the boiling point is 2732 °C.
- Atomic Number of Nickel Atomic Number of Nickel is 28. Chemical symbol for Nickel is Ni. Number of protons in Nickel is 28.
- Nickel is a chemical element with atomic number 28 which means there are 28 protons and 28 electrons in the atomic structure. The chemical symbol for Nickel is Ni. Electron Configuration and Oxidation States of Nickel Electron configuration of Nickel is Ar 3d8 4s2.
About Nickel
Nickel is a light grey color metal, which is quite strong and very resistive to corrosion. The name of the element is believed to take roots from the German expression meaning Devil’s copper. Despite the fact that nickel can be found in cells of all living organisms, this element is very carcinogenic even in small doses. It is impossible to find pure nickel on our planet, only in minerals, as well as on meteorites coming to our planet (one of them has landed in Canada and now provides about 15 per cent of the world’s nickel supply). Nickel used to be a key element for producing coins, and it is also used to produce batteries. Due its high resistance to corrosion, nickel is used to cover various metallic parts in order to prevent their corrosion. Numerous alloys of nickel have wide industrial uses.
Properties of Nickel Element
| Atomic Number (Z) | 28 |
|---|---|
| Atomic Symbol | Ni |
| Group | 10 |
| Period | 4 |
| Atomic Weight | 58.6934 u |
| Density | 8.912 g/cm3 |
| Melting Point (K) | 1728 K |
| Melting Point (℃) | 1453 °C |
| Boiling Point (K) | 3186 K |
| Boiling Point (℃) | 2732 °C |
| Heat Capacity | 0.444 J/g · K |
| Abundance | 84 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 1.88 |
| Ionization Energy (eV) | 7.881 |
| Atomic Radius | 135pm |
| Covalent Radius | 126pm |
| Valence Electrons | 2 |
| Year of Discovery | 1751 |
| Discoverer | Cronstedt |
What is the Boiling Point of Nickel?
Nickel boiling point is 2732 °C. Boiling point of Nickel in Kelvin is 3186 K.
What is the Melting Point of Nickel?
Nickel melting point is 1453 °C. Melting point of Nickel in Kelvin is 1728 K.
How Abundant is Nickel?
Abundant value of Nickel is 84 mg/kg.
What is the State of Nickel at Standard Temperature and Pressure (STP)?
State of Nickel is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Nickel Discovered?
Nickel was discovered in 1751.
A
$Cu^{+}$
B$Cu^{2+}$
C$Ni^{2+}$
D$Ni$
Solution:
Electronic configuration of$_{28}Ni = Is^2, 2s^2 2p^6, 3s^23p^63d^8, 4s^2$
$ Ni^{2+} = Is^2, 2s^22p^6, 3s^2 3p^6 3d^8, 4s^0$
$_{29}Cu = 1s^2 ,2s^2p^6 ,3s^23p^63d^{10} ,4s^1$
$Cu^+ = Is^2, 2s^22p^6, 3s^23p^63d^{10}, 4s^0$
So, the given configuration is of $ Cu^+$
1. The formation of $ O^{+}_2 [P_t F_6 ]^-$ is the basis for the formation of Xenon fluorides. This is because
2. The highest magnetic moment is shown by the transition metal ion with the configuration
3. In which of the following complex ion, the central metal ion is in a state of $sp^3d^2$ hybridisation ?
4. Which of the following can participate in linkage isomerism ?
5. Which of the following has the highest bond order ?
6. Which of the following is diamagnetic ?
7. In the following, the element with the highest ionisation energy is
8. In the conversion of $Br_2$ to $BrO^-_3 $, the oxidation number of Br changes from
9. Among the alkali metals cesium is the most reactive because
10. Which of the following represents the Lewis structure of $N_2$ molecule ?
1. The following nuclear transmission
$ce{^{23}_{11}Na + ^{1}_{1} H to ^{23}_{12} Mg + ^{1}_{0} n}$
belongs to
2. For emission line of atomic hydrogen from $n_i = 8$ to $n_f $ = the plot of wave number $(bar{v})$ against $( frac{1}{n^2})$ will be (The Ry dbergconstant, $R_H$ is in wave number unit).
3. Half-life of radium is 1580 years. Its average life will be
4. Which of the following arrangement is possible?
$quad$$quad$n$quad$ $quad$l$quad$$quad$m$quad$$quad$s
5. Quantum numbers of an atom can be defined on the basis of

6. Spectrum of Li2+ is similar to that of
7. Azimuthal quantum number defines
8. The quantum number m of a free gaseous atom is associated with
9. The isoelectronic pair is
10. For principle quantum number n = 4, the total number of orbitals having l = 3 is
1. In Wolff‐Kishner reduction, the carbonyl group of aldehydes and ketones is converted into
Atomic Number Of Nitrogen-15
2. Identify compound X in the following sequence of reactions:
3. Identify a molecule which does not exist.
4. Identify the incorrect match.
Name IUPAC Official Name A Unnilunium i Mendelevium B Unniltrium ii Lawrencium C Unnilhexium iii Seaborgium D Unununnium iv Darmstadtium
| Name | IUPAC Official Name | ||
|---|---|---|---|
| A | Unnilunium | i | Mendelevium |
| B | Unniltrium | ii | Lawrencium |
| C | Unnilhexium | iii | Seaborgium |
| D | Unununnium | iv | Darmstadtium |
5. Reaction between acetone and methyl magnesium chloride followed by hydrolysis will give :
6. Identify the correct statements from the following:
(a) $CO_2(g)$ is used as refrigerant for ice-cream and frozen food.
(b) The structure of $C_{60}$ contains twelve six carbon rings and twenty five carbon rings.
(c) $ZSM-5$, a type of zeolite, is used to convert alcohols into gasoline.
(d) $CO$ is colorless and odourless gas.
7. Which of the following set of molecules will have zero dipole moment ?
8. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be:
Atomic Number Of Nitrogen Crossword Clue
9. An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. The atomic radiusis:
Atomic Number Of Nitrate
10. Find out the solubility of $Ni(OH)_2$ in 0.1 M NaOH. Given that the ionic product of $Ni(OH)_2$ is $2 times 10^{-15}$
